Interfacial adsorption of pH-responsive polymers and nanoparticles†
Abstract
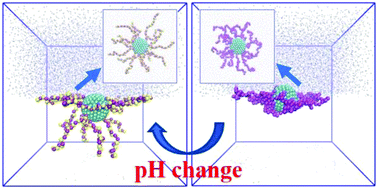
Using dissipative particle dynamics (DPD), we model the interfacial adsorption of pH-responsive polyelectrolytes and polyelectrolyte-grafted nanoparticles (PNPs) at a planar water–oil interface. The electrostatic interactions in the presence of the dielectric discontinuity across the interface are modeled by exploiting the Groot method, which uses an iterative method to solve the Poisson equation on a uniform grid with distributed charge. We reveal the effects of the pH and salinity of the aqueous solution and the length of the polyelectrolyte on the adsorption behavior of weak polyelectrolytes. The adsorption kinetics is monitored via the trajectory of the center of mass of the polyelectrolyte in the direction normal to the interface. The residence time at the interface and the pair correlation function between the polyelectrolyte and the oil are measured to quantitatively characterize the adsorption. Similar to the weak polyelectrolytes, the influences of pH, salinity and grafted chain length on the adsorption of an individual PNP are explored. Our results show that by grafting polyelectrolytes, the interfacial behavior of the nanoparticles can be tuned by changing the pH and salinity of the solution, which is dictated by the contact angle, the pair correlation function between the particles and the oil, the desorption energy, and the particle morphology at the interface. We also observe that the electrostatic-driven variations in the interfacial activity and morphology of the PNPs are not sensitive to the length of the grafted polyelectrolytes.


 Please wait while we load your content...
Please wait while we load your content...