Lipid rafts enhance the binding constant of membrane-anchored receptors and ligands
Abstract
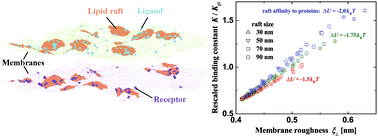
Gaining insights into the binding of membrane-anchored receptors and ligands that mediate cell adhesion and signal transduction is of great significance for understanding numerous physiological processes driven by intercellular communication. Lipid rafts, microdomains in cell membranes enriched in cholesterol and saturated lipids such as sphingomyelin, are believed to serve as the essential platforms to recruit protein molecules for biological functions. An important question remains how the lipid rafts affect the binding constant of membrane-anchored receptors and ligands. We have investigated the adhesion of multicomponent membranes by using Monte Carlo simulations of a mesoscopic model with biologically relevant parameters. We find that the preferential partitioning of membrane-anchored receptor and ligand proteins in the lipid rafts significantly increases the binding constant of those proteins, in cooperation with the shape fluctuations of the membranes caused by thermal excitations. The binding constant can even be greater than that of the same receptors and ligands anchored to two apposing supported, planar membranes without shape fluctuations. The membrane shape fluctuations facilitate the binding of the anchored receptors and ligands, in contrast to the case of homogeneous membranes. Our results suggest that cells might regulate the binding of membrane-anchored receptor and ligand proteins by modulating the properties of lipid rafts such as area fraction, size and the affinity of rafts to the proteins.


 Please wait while we load your content...
Please wait while we load your content...