Heterogeneity is key to hydrogel-based cartilage tissue regeneration
Abstract
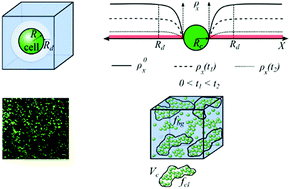
Degradable hydrogels have been developed to provide initial mechanical support to encapsulated cells while facilitating the growth of neo-tissues. When cells are encapsulated within degradable hydrogels, the process of neo-tissue growth is complicated by the coupled phenomena of transport of large extracellular matrix macromolecules and the rate of hydrogel degradation. If hydrogel degradation is too slow, neo-tissue growth is hindered, whereas if it is too fast, complete loss of mechanical integrity can occur. Therefore, there is a need for effective modelling techniques to predict hydrogel designs based on the growth parameters of the neo-tissue. In this article, hydrolytically degradable hydrogels are investigated due to their promise in tissue engineering. A key output of the model focuses on the ability of the construct to maintain overall structural integrity as the construct transitions from a pure hydrogel to engineered neo-tissue. We show that heterogeneity in cross-link density and cell distribution is the key to this successful transition and ultimately to achieve tissue growth. Specifically, we find that optimally large regions of weak cross-linking around cells in the hydrogel and well-connected and dense cell clusters create the optimum conditions needed for neo-tissue growth while maintaining structural integrity. Experimental observations using cartilage cells encapsulated in a hydrolytically degradable hydrogel are compared with model predictions to show the potential of the proposed model.


 Please wait while we load your content...
Please wait while we load your content...