Comment on “Relating side chain organization of PNIPAm with its conformation in aqueous methanol” by D. Mukherji, M. Wagner, M. D. Watson, S. Winzen, T. E. de Oliveira, C. M. Marques and K. Kremer, Soft Matter, 2016, 12, 7995
Abstract
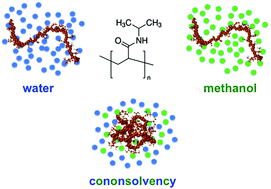
In a recent paper, Mukherji et al. describe the collapse of poly(N-isopropyl acrylamide) in methanol–water mixtures based on experiments and molecular dynamics simulations. The conclusion of their work is that chain collapse is dominated by enthalpic bridging interactions while entropic effects play no major role. Here we show that this claim arises from an improper interpretation of preferential binding and the corresponding thermodynamic data presented. When interpreted correctly, the data instead provide evidence for repulsive enthalpic interactions of methanol with the polymer, supporting the emerging view of entropic chain collapse.

 Please wait while we load your content...
Please wait while we load your content...