A broad parameter range for selective methane production with bicarbonate solution in electrochemical CO2 reduction†
Abstract
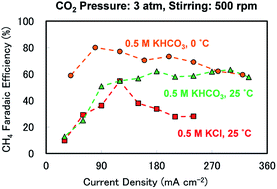
This study demonstrated the previously unrecognised capability of potassium bicarbonate (KHCO3) aqueous solutions to assist in the selective generation of methane (CH4) over a wide range of reaction parameters during electrochemical CO2 reduction over a bulk polycrystalline Cu catalyst. Compared with the results obtained with potassium chloride (KCl) electrolytes, a 0.5 M KHCO3 solution maintained a higher faradaic efficiency (FE) for CH4 production at higher current densities. This trend was further emphasised upon increasing the CO2 pressure from near-ambient to 3 atm and incorporating stirring. The result was a FE above 50% over a range of current densities from 90 to more than 330 mA cm−2. The maximum FE reached 80% at 0 °C, while maintaining a broad peak structure when plotted against current density, even over 300 mA cm−2. The buffering ability of KHCO3 appears to play an important role in increasing both the reaction rate and the selectivity for CH4, especially when combined with an optimised CO2 supply to the electrode and an ideal reaction temperature.


 Please wait while we load your content...
Please wait while we load your content...