An investigation of Cu–Re–ZnO catalysts for the hydrogenolysis of glycerol under continuous flow conditions†
Abstract
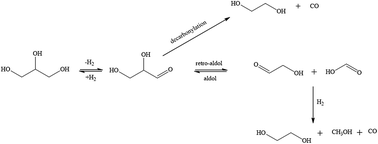
Cu and Re monometallic and bimetallic catalysts supported on ZnO were synthesized via wet impregnation. The catalysts were characterized using XRD, TPR, Pulse TPD, TEM, SEM, XPS and BET surface area. TPR results showed that the presence of rhenium increases the reduction temperature of the catalysts and TPD showed that the presence of copper decreases the Brønsted acidity of the catalysts. SEM showed an improved distribution of metal oxide on the support after the incorporation of rhenium. These catalysts were evaluated in the hydrogenolysis of glycerol in a continuous flow fixed bed reactor in a temperature range of 150–250 °C and a H2 pressure of 60 bar. All catalysts were active, with activity being higher over the rhenium containing catalysts. At the lowest temperature (150 °C), 1,2-propanediol had the highest selectivity which decreased with increase in temperature. Subsequently, the selectivity to lower alcohols, such as methanol, ethanol and 1-propanol, and ethylene glycol increased with temperature as 1,2-propanediol reacted further to these products due to C–C bond cleavage. This was also observed when the hydrogen content was increased at constant temperature (250 °C). All catalysts were found to be stable in terms of activity and selectivity to lower alcohols over a period of at least 24 hours at 250 °C and 60 bar H2 pressure.


 Please wait while we load your content...
Please wait while we load your content...