Automatic high-pressure hydrogen generation from formic acid in the presence of nano-Pd heterogeneous catalysts at mild temperatures†
Abstract
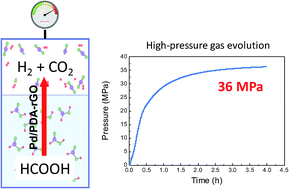
High-pressure hydrogen is of great interest in the industrial utilization of hydrogen energy, especially for hydrogen fuel cell vehicles. In this work, a method of automatic high-pressure H2 generation by the decomposition of formic acid, a recently renowned hydrogen storage material, in the presence of a heterogeneous catalyst (palladium nano-particles on a graphene oxide catalyst (Pd/PDA–rGO)) was proposed. This catalyst can effectively catalyze the decomposition of formic acid to produce high-pressure H2 and CO2 over 35 MPa without any detectable formation of CO or other by-products. For example, a 36.3 MPa total gas pressure was successfully achieved using an aqueous solution of 6.7 mol L−1 formic acid and 6.7 mol L−1 sodium formate at 80 °C. This research provided a preliminary study on the automatic high-pressure hydrogen gas generation by the decomposition of formic acid without any compression facilities in the presence of a heterogeneous catalyst, which can be easily separated from the reaction process, for hydrogen energy utilization.


 Please wait while we load your content...
Please wait while we load your content...