Destabilisation of Ca(BH4)2 and Mg(BH4)2via confinement in nanoporous Cu2S hollow spheres†
Abstract
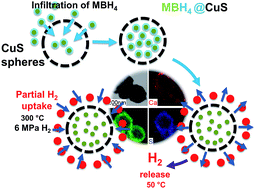
Complex borohydrides of calcium (Ca(BH4)2) and magnesium (Mg(BH4)2) have gained increasing attention as promising hydrogen storage materials due to their high volumetric and gravimetric density. However, these borohydrides suffer from high desorption temperatures and poor hydrogen reversibility. In this study, Ca(BH4)2 and Mg(BH4)2 were confined within the porosity of Cu2S hollow spheres. Upon confinement, hydrogen was released at a temperature as low as 50 °C and full hydrogen release was completed by 300 °C. Upon cycling at 300 °C and 6 MPa H2 pressure, some hydrogen uptake of 0.9 and 0.5 mass% was achieved for these composite materials, i.e. Ca(BH4)2@Cu2S and Mg(BH4)2@Cu2S, respectively. Partial rehydrogenation was confirmed by FTIR proving the regeneration of borohydride phases. The nature of these improvements was studied through comparison to the physical mixture of borohydrides and Cu2S hollow spheres, and the results confirmed the effect of nanoconfinement in facilitating the destabilisation of Ca(BH4)2 and Mg(BH4)2. These results confirm that a well-designed nanoporous structure confining the decomposition products of borohydrides during hydrogen release could potentially lead to high reversible hydrogen storage material.
- This article is part of the themed collection: 2017 Sustainable Energy and Fuels HOT Articles


 Please wait while we load your content...
Please wait while we load your content...