A study on the nature of the thermal decomposition of methylammonium lead iodide perovskite, CH3NH3PbI3: an attempt to rationalise contradictory experimental results
Abstract
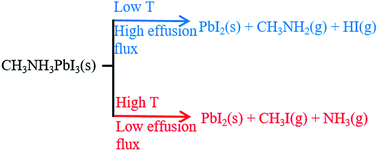
The nature of the gas phase product released during the thermal decomposition of CH3NH3PbI3 (methylammonium lead iodide) to PbI2 (lead diiodide) under vacuum is discussed on the basis of thermodynamic predictions, recently published experimental results, and new experiments presented here. From the limited data currently available, the nature of the main decomposition path is not clear because, both, the process releasing HI(g) + CH3NH2(g) (1) and that leading to NH3(g) + CH3I(g) (2) were observed under different conditions. Our thermodynamic analysis showed that process (2) is largely favoured for all the CH3NH3PbX3 (X = Cl, Br, I) compounds. However, Knudsen effusion mass spectrometry experiments (temperature range 140–240 °C) showed that HI(g) and CH3NH2(g) were the predominant species in the vapor, with process (2) occurring to a much smaller extent than suggested by the thermodynamic driving force, thus being of minor importance under effusion conditions. We also found that this process was comparatively enhanced by high temperatures and low effusion rates (high impedance orifice). Our experimental evidence suggested that the thermodynamically favoured process (2) was affected by a significant kinetic hindrance. Overall, the prevailing decomposition path is likely to markedly depend on the actual operative conditions.


 Please wait while we load your content...
Please wait while we load your content...