Iron oxide deposited on metallic nickel for water oxidation†
Abstract
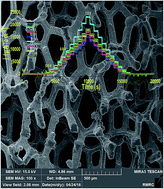
Solar energy is too intermittent to be directly used on a large scale, and thus, a large energy storage system should be developed. One interesting approach is the use of sunlight to perform water splitting for hydrogen production. For such systems, developing an efficient and stable water-oxidizing catalyst is an essential task. Using K2FeO4, we report a simple method to deposit iron oxide on metallic nickel for water oxidation. The Fe/Ni-based electrode, at pH = 13, shows a current density of 1.9 mA cm−2 at an overpotential of 250.0 mV, making it a promising anode for use in water-splitting systems. At a higher overpotential (700.0 mV), a current density of 23.5 mA cm−2 was observed.


 Please wait while we load your content...
Please wait while we load your content...