Proton transfer in microbial electrolysis cells†
Abstract
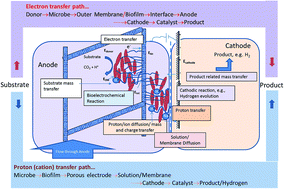
Proton transfer and electron transfer are of prime importance in the development of microbial electrochemical cells. While electron transfer is primarily controlled by biology, proton transfer is controlled by process engineering and cell design. To develop commercially feasible technologies around the concept of a bioelectrochemical cell, real feedstocks have to be explored and associated limitations have to be identified. In this study, the proton transfer rate was quantified for a microbial electrolysis cell (MEC) and its dependence on process parameters was investigated using a proton balance model. The reaction system consisted of a biomass-derived pyrolytic aqueous stream as a substrate producing hydrogen in a flow-through MEC. The proton transfer rate increased with anode flow rate and organic loading rate up to a maximum of 0.36 ± 0.01 moles per m2 per h, equivalent to a hydrogen production rate of 9.08 L per L per day. Higher rates of hydrogen production, reaching 11.7 ± 0.2 L per L per day were achieved, when additional protons were provided via the cathode buffer. Electrochemical impedance spectroscopy shows that proton transfer was the dominant resistance in the production of hydrogen. The quantification of proton transfer rates for MECs with potential for biorefinery application and the demonstration of high hydrogen production rates approaching those required for commercial consideration indicate the strong potential of this technology for renewable hydrogen production. Understanding the transport phenomenon in bioelectrochemical cells is of great significance since these systems have potential for wide-ranging applications including energy production, bioremediation, chemical and nanomaterial synthesis, electro-fermentation, energy storage, desalination, and produced water treatment. Electron transfer in anode biofilms has been investigated extensively, but proton transfer studies are also important, since many cathodic half reactions require protons as the reactant. Determination of transport rates via proton balance was investigated in microbial electrolysis cells, which can be applied to other forms of microbial electrochemical systems. These systems have a unique niche in the development of future biorefineries as a means of recovering energy from waste streams with potential for water recycle, making them an integral part of the water–energy nexus focus area.


 Please wait while we load your content...
Please wait while we load your content...