CeO2@C derived from benzene carboxylate bridged metal–organic frameworks: ligand induced morphology evolution and influence on the electrochemical properties as a lithium-ion battery anode†
Abstract
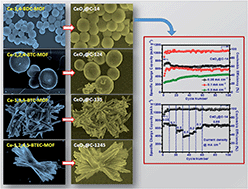
We report herein a facile metal–organic framework (MOF) derived route for the synthesis of carbon embedded CeO2 (CeO2@C) with a pre-designed shape-specific morphology by varying the organic linker and by using PVP as the structure directing agent. It is found that the general morphological features of the parent MOF are mimicked by the derived oxide. Four different linkers have been used to prepare CeO2@C particles with three different shapes – spherical, bar-shaped and thin plate-like. A probable formation mechanism is discussed based on metal–ligand coordination. Influence of the morphology on the electrochemical properties as a lithium-ion battery (LIB) anode has been studied in coin cells vs. Li/Li+. The spherically shaped CeO2@C-14 shows a superior performance with a maximum specific capacity of 715 mA h g−1 at 0.05 mA cm−2, good rate performance (413 mA h g−1 at 0.5 mA cm−2) and cycling stability (∼94% capacity retention after 100 cycles). The present results demonstrate that the major limitations of metal oxide anodes – volume expansion during lithiation/delithiation, rate performance and capacity fading upon cycling – could be overcome to a great extent by adopting the two-way approach of morphology design through the MOF route and in situ embedded carbon matrix.
- This article is part of the themed collection: 2017 Sustainable Energy and Fuels HOT Articles


 Please wait while we load your content...
Please wait while we load your content...