Guiding a divergent reaction by photochemical control: bichromatic selective access to levulinates and butenolides†
Abstract
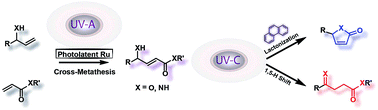
Allylic and acrylic substrates may be efficiently transformed by a sequential bichromatic photochemical process into derivatives of levulinates or butenolides with high selectivity when phenanthrene is used as a regulator. Thus, UV-A photoinduced cross-metathesis (CM) couples the acrylic and allylic counterparts and subsequent UV-C irradiation initiates E–Z isomerization of the carbon–carbon double bond, followed by one of two competing processes; namely, cyclization by transesterification or a 1,5-H shift and tautomerization. Quantum chemical calculations demonstrate that intermediates are strongly blue-shifted for the cyclization while red-shifted for the 1,5-H shift reaction. Hence, delaying the double bond migration by employing UV-C absorbing phenanthrene, results in a selective novel divergent all-photochemical pathway for the synthesis of fundamental structural motifs of ubiquitous natural products.


 Please wait while we load your content...
Please wait while we load your content...