Designing interactions by control of protein–ligand complex conformation: tuning arginine–arene interaction geometry for enhanced electrostatic protein–ligand interactions†
Abstract
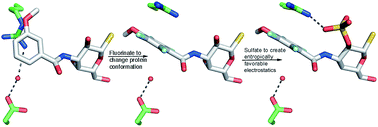
We investigated galectin-3 binding to 3-benzamido-2-O-sulfo-galactoside and -thiodigalactoside ligands using a combination of site-specific mutagenesis, X-ray crystallography, computational approaches, and binding thermodynamics measurements. The results reveal a conformational variability in a surface-exposed arginine (R144) side chain in response to different aromatic C3-substituents of bound galactoside-based ligands. Fluorinated C3-benzamido substituents induced a shift in the side-chain conformation of R144 to allow for an entropically favored electrostatic interaction between its guanidine group and the 2-O-sulfate of the ligand. By contrast, binding of ligands with non-fluorinated substituents did not trigger a conformational change of R144. Hence, a sulfate–arginine electrostatic interaction can be tuned by the choice of ligand C3-benzamido structures to favor specific interaction modes and geometries. These results have important general implications for ligand design, as the proper choice of arginine–aromatic interacting partners opens up for ligand-controlled protein conformation that in turn may be systematically exploited in ligand design.


 Please wait while we load your content...
Please wait while we load your content...