Stereoselective construction of sterically hindered oxaspirocycles via chiral bidentate directing group-mediated C(sp3)–O bond formation†
Abstract
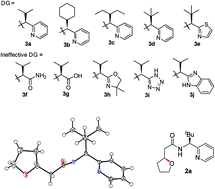
The systematic investigation of chiral bidentate auxiliaries has resulted in the discovery of a chiral 2,2-dimethyl-1-(pyridin-2-yl)propan-1-amine-derived directing group that enables stereoselective palladium(II)-catalyzed intramolecular C(sp3)–O bond formation. This new chiral directing group exhibited high reactivity in the activation of methylene C(sp3)–H bonds with excellent levels of stereoselectivity (a diastereomeric ratio of up to 39 : 1), which allowed the construction of a wide range of oxaspirocycles. Mechanistic investigations were also conducted to elucidate the reaction mechanism and understand the origin of the diastereoselectivity. DFT calculations suggest that only modest levels of diastereoselectivity are accomplished at the rate-determining C–H metalation–deprotonation step and the d.r. is further enriched at the reductive elimination step.


 Please wait while we load your content...
Please wait while we load your content...