Observing enzyme ternary transition state analogue complexes by 19F NMR spectroscopy†
Abstract
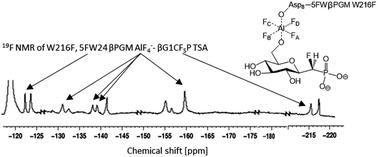
Ternary transition state analogue (TSA) complexes probing the isomerization of β-D-glucose 1-phosphate (G1P) into D-glucose 6-phosphate (G6P) catalyzed by catalytically active, fluorinated (5-fluorotryptophan), β-phosphoglucomutase (βPGM) have been observed directly by 19F NMR spectroscopy. In these complexes MgF3− and AlF4− are surrogates for the transferring phosphate. However, the relevance of these metal fluorides as TSA complexes has been queried. The 1D 19F spectrum of a ternary TSA complex presented a molar equivalence between fluorinated enzyme, metal fluoride and non-isomerizable fluoromethylenephosphonate substrate analogue. Ring flips of the 5-fluoroindole ring remote from the active site were observed by both 19F NMR and X-ray crystallography, but did not perturb function. This data unequivocally demonstrates that the concentration of the metal fluoride complexes is equivalent to the concentration of enzyme and ligand in the TSA complex in aqueous solution.


 Please wait while we load your content...
Please wait while we load your content...