Gold-catalyzed stereoselective dearomatization/metal-free aerobic oxidation: access to 3-substituted indolines/oxindoles†
Abstract
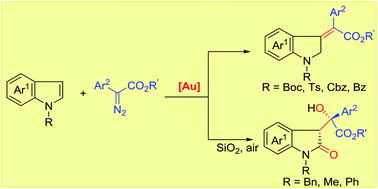
An unprecedented dearomatization of indoles with diazoesters has been developed via cationic gold(I) catalysis. The functionalization selectively occurs at the C3-position to deliver methylene indole derivatives in good yields with excellent Z-selectivity, demonstrating unusual reactivity and selectivity compared with other noble metal catalysis. Importantly, simply followed by silica gel adsorption, an unprecedented metal-free aerobic oxidation occurs for indoles bearing N-electron donating substituents, providing a novel and efficient approach towards 3-substituted indolin-2-ones with a newly formed quaternary stereocenter in excellent stereoselectivity. Notably, these processes afford direct and selective access to a variety of valuable intermediates from abundant feedstock chemicals.


 Please wait while we load your content...
Please wait while we load your content...