Catalytic 1,2-dihydronaphthalene and E-aryl-diene synthesis via CoIII–Carbene radical and o-quinodimethane intermediates†
Abstract
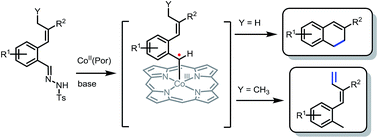
Catalytic synthesis of substituted 1,2-dihydronaphthalenes via metalloradical activation of o-styryl N-tosyl hydrazones ((E)-2-(prop-1-en-1-yl)benzene-N-tosyl hydrazones) is presented, taking advantage of the intrinsic reactivity of a cobalt(III)–carbene radical intermediate. The method has been successfully applied to a broad range of substrates with various R1 substituents at the aromatic ring, producing the desired ring products in good to excellent isolated yields for substrates with an R2 = COOEt substituent at the vinylic position (∼70–90%). Changing the R2 moiety from an ester to other substituents has a surprisingly large influence on the (isolated) yields. This behaviour is unexpected for a radical rebound ring-closure mechanism, and points to a mechanism proceeding via ortho-quinodimethane (o-QDM) intermediates. Furthermore, substrates with an alkyl substituent on the allylic position reacted to form E-aryl-dienes in excellent yields, rather than the expected 1,2-dihydronaphthalenes. This result, combined with the outcome of supporting DFT calculations, strongly points to the release of reactive o-QDM intermediates from the metal centre in all cases, which either undergo a 6π-cyclisation step to form the 1,2-dihydronaphthalenes, or a [1,7]-hydride shift to produce the E-aryl-dienes. Trapping experiments using TEMPO confirm the involvement of cobalt(III)–carbene radical intermediates. EPR spectroscopic spin-trapping experiments using phenyl N-tert-butylnitrone (PBN) confirm the radical nature of the catalytic reaction.
- This article is part of the themed collection: International Symposium on Macrocyclic & Supramolecular Chemistry (ISMSC) in conjunction with ISACS


 Please wait while we load your content...
Please wait while we load your content...