Bioinspired synthesis of pentacyclic onocerane triterpenoids†
Abstract
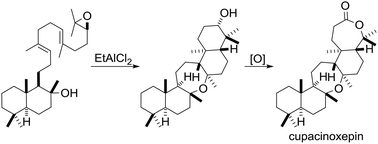
The first chemical synthesis of pentacyclic onocerane triterpenoids has been achieved. A putative biomimetic tricyclization cascade is employed to forge a fused decalin-/oxepane ring system. The synthetic route proceeds to (+)-cupacinoxepin in seven steps and to (+)-onoceranoxide in eight steps in the longest linear sequence, when starting from geranyl chloride and (+)-sclareolide. The bioinspired epoxypolyene cyclization is supported by computational and enzymatic studies.


 Please wait while we load your content...
Please wait while we load your content...