Exploiting rhodium-catalysed ynamide hydroacylation as a platform for divergent heterocycle synthesis†
Abstract
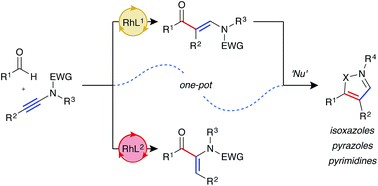
The first examples of ynamide hydroacylation are described. Using rhodium catalysis, linear β-enaminone products are generated in high yield and excellent regioselectivity from the combination of aldehydes and ynamides. The enaminone products are subsequently used as a platform to construct a diverse array of substituted pyrazoles, pyrimidines, and isoxazoles in a two-step, one-pot sequence. It was found that with judicious choice of catalyst system it was possible to overturn the regioselectivity of the hydroacylation reaction to generate α-enaminone products.


 Please wait while we load your content...
Please wait while we load your content...