Evolved polymerases facilitate selection of fully 2′-OMe-modified aptamers†
Abstract
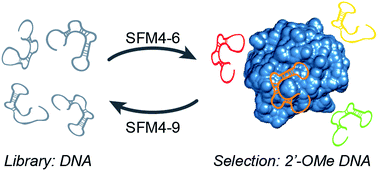
RNA or DNA aptamers with 2′-OMe-modifications have been pursued to increase resistance to nucleases, but have been difficult to identify because the OMe groups ablate polymerase recognition. We recently reported evolution of the thermostable DNA polymerases SFM4-6 and SFM4-9, which enable the efficient “transcription” and “reverse transcription”, respectively, of 2′-OMe oligonucleotides. With these polymerases, we now report the first selection of fully 2′-OMe modified aptamers, specifically aptamers that bind human neutrophil elastase (HNE). Two aptamers, 2mHNE-1 and 2mHNE-2, were isolated after five rounds of selection, and four more, 2mHNE-3–6, after an additional five rounds that included selection pressure for binding in the presence of serum. All six aptamers bind with reasonable affinity, which requires the 2′-OMe substituents. Further characterization of one aptamer, 2mHNE-5, showed that unlike a previously reported natural anti-HNE aptamer, affinity for HNE is retained in the presence of high concentrations of salt or serum. The polymerases SFM4-6 and SFM4-9 should prove valuable for the production and further exploration of modified aptamers.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners


 Please wait while we load your content...
Please wait while we load your content...