Synthesis of a terminal Ce(iv) oxo complex by photolysis of a Ce(iii) nitrate complex†
Abstract
Reaction of [Ce(NR2)3] (R = SiMe3) with LiNO3 in THF, in the presence of 2,2,2-cryptand, results in the formation of the Ce(III) “ate” complex, [Li(2,2,2-cryptand)][Ce(κ2-O2NO)(NR2)3] (1) in 38% yield. Photolysis of 1 at 380 nm affords [Li(2,2,2-cryptand)][Ce(O)(NR2)3] (2), in 33% isolated yield after reaction work-up. Complex 2 is the first reported example of a Ce(IV) oxo complex where the oxo ligand is not supported by hydrogen bonding or alkali metal coordination. Also formed during photolysis are [Li(2,2,2-cryptand)]2[(μ3-O){Ce(μ-O)(NR2)2}3] (3) and [Li(2,2,2-cryptand)][Ce(OSiMe3)(NR2)3] (4). Their identities were confirmed by X-ray crystallography. Complex 4 can also be prepared via reaction of [Ce(NR2)3] with LiOSiMe3 in THF, in the presence of 2,2,2-cryptand. When synthesized in this fashion, 4 can be isolated in 47% yield. To rationalize the presence of 2, 3, and 4 in the reaction mixture, we propose that photolysis of 1 first generates 2 and NO2, via homolytic cleavage of the N–O bond in its nitrate co-ligand. Complex 2 then undergoes decomposition via two separate routes: (1) ligand scrambling and oligomerization to form 3; and, (2) abstraction of a trimethylsilyl cation to form a transient Ce(IV) silyloxide, [CeIV(OSiMe3)(NR2)3], followed by 1e− reduction to form 4. Alternatively, complex 4 could form directly via ·SiMe3 abstraction by 2.
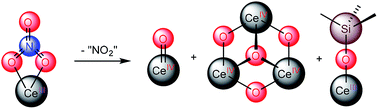


 Please wait while we load your content...
Please wait while we load your content...