Fluorogenic labeling and single-base resolution analysis of 5-formylcytosine in DNA†
Abstract
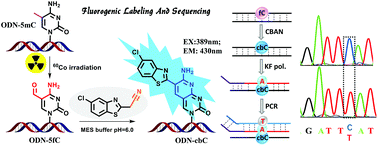
5-Formylcytosine (5fC), which plays an important role in epigenetic functions, has received widespread attention in many related fields. Here, we demonstrate a new design for both the fluorogenic switch-on detection and single-base resolution analysis of 5fC through selectively reacting a reagent with 5fC to yield an intramolecular cyclization nucleobase. The generated product, bearing a similar benzothiazole-iminocoumarin scaffold, is highly fluorescent and enables us to qualitatively and quantitatively detect 5fC moieties in γ-irradiated calf thymus DNA. Additionally, losing the exocyclic 4-amino group in 5fC causes the incorporation of dATP through base pairing with the generated nucleobase during polymerase extension, which helped us to analyze the 5fC sites in both single- and double-stranded oligonucleotides. Our Sanger and Illumina sequencing results show great potential in single-base resolution analysis of 5fC. It is hopeful that a similar design may be used for more detection targets.


 Please wait while we load your content...
Please wait while we load your content...