Tertiary amine synthesis via reductive coupling of amides with Grignard reagents†
Abstract
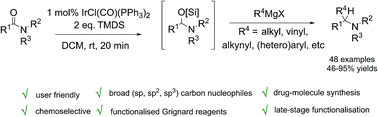
A new iridium catalyzed reductive coupling reaction of Grignard reagents and tertiary amides affording functionalised tertiary amine products via an efficient and technically-simple one-pot, two-stage experimental protocol, is reported. The reaction – which can be carried out on gram-scale using as little as 1 mol% Vaska's complex [IrCl(CO)(PPh3)2] and TMDS as the terminal reductant for the initial reductive activation step – tolerates a broad range of tertiary amides from (hetero)aromatic to aliphatic (branched, unbranched and formyl) and a wide variety of alkyl (linear, branched), vinyl, alkynyl and (hetero)aryl Grignard reagents. The new methodology has been applied directly to bioactive molecule synthesis and the high chemoselectivity of the reductive coupling of amide has been exploited in late stage functionalization of drug molecules. This reductive functionalisation of tertiary amides provides a new and practical solution to tertiary amine synthesis.


 Please wait while we load your content...
Please wait while we load your content...