All-metal aromatic cationic palladium triangles can mimic aromatic donor ligands with Lewis acidic cations†
Abstract
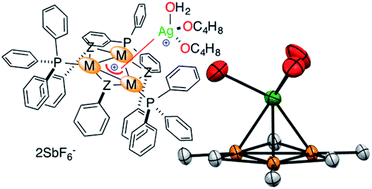
We present that cationic rings can act as donor ligands thanks to suitably delocalized metal–metal bonds. This could grant parent complexes with the peculiar properties of aromatic rings that are crafted with main group elements. We assembled Pd nuclei into equilateral mono-cationic triangles with unhindered faces. Like their main group element counterparts and despite their positive charge, these noble-metal rings form stable bonding interactions with other cations, such as positively charged silver atoms, to deliver the corresponding tetranuclear dicationic complexes. Through a mix of modeling and experimental techniques we propose that this bonding mode is an original coordination-like one rather than a 4-centre–2-electron bond, which have already been observed in three dimensional aromatics. The present results thus pave the way for the use of suitable metal rings as ligands.


 Please wait while we load your content...
Please wait while we load your content...