Copper-catalyzed aminoalkynylation of alkenes with hypervalent iodine reagents†
Abstract
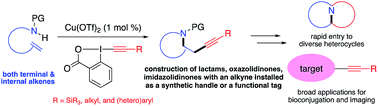
A copper-catalyzed aminoalkynylation of alkenes is achieved with ethynylbenziodoxolone (EBX) reagents under mild conditions with only 1 mol% copper catalyst. This transformation allows for rapid construction of diverse important azahetereocycles and installation of valuable alkyne groups in one step. The developed method features remarkable substrate scope for both terminal and internal alkenes as well as different alkynyl groups, presenting great potential for broad applications in synthesis, bioconjugation, and molecular imaging.


 Please wait while we load your content...
Please wait while we load your content...