Enantioselective synthesis of gem-diarylalkanes by transition metal-catalyzed asymmetric arylations (TMCAAr)
Abstract
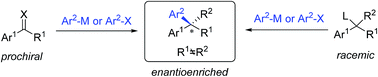
Chiral gem(1,1)-diaryl containing tertiary or quaternary stereogenic centers are present in many natural products and important pharmacophores. While numerous catalytic asymmetric methods enable access to 1,1-diaryl motifs, transition metal-catalyzed asymmetric arylations (TMCAAr) are one of the most powerful methods to prepare enantiopure gem-diarylalkane compounds. The main methodology includes enantioselective 1,2- or 1,4-additions across C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds by arylmetallic reagents; aryl cross-couplings of olefins, benzylic (pseudo)halides and aziridines; asymmetric aryl substitution reactions of allylic substrates; and isotopic benzylic C–H arylation.
C bonds by arylmetallic reagents; aryl cross-couplings of olefins, benzylic (pseudo)halides and aziridines; asymmetric aryl substitution reactions of allylic substrates; and isotopic benzylic C–H arylation.
- This article is part of the themed collection: Most popular 2018-2019 catalysis articles


 Please wait while we load your content...
Please wait while we load your content...