Effects of electron transfer on the stability of hydrogen bonds†
Abstract
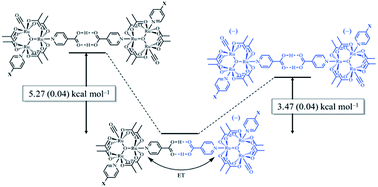
The measurement of the dimerization constants of hydrogen-bonded ruthenium complexes (12, 22, 32) linked by a self-complementary pair of 4-pyridylcarboxylic acid ligands in different redox states is reported. Using a combination of FTIR and UV/vis/NIR spectroscopies, the dimerization constants (KD) of the isovalent, neutral states, 12, 22, 32, were found to range from 75 to 130 M−1 (ΔG0 = −2.56 to −2.88 kcal mol−1), while the dimerization constants (K2−) of the isovalent, doubly-reduced states, (12)2−, (22)2−, (32)2−, were found to range from 2000 to 2500 M−1 (ΔG0 = −4.5 to −4.63 kcal mol−1). From the aforementioned values and the comproportionation constant for the mixed-valent dimers, the dimerization constants (KMV) of the mixed-valent, hydrogen-bonded dimers, (12)−, (22)−, (32)−, were found to range from 0.5 × 106 to 1.2 × 106 M−1 (ΔG0 = −7.78 to −8.31 kcal mol−1). On average, the hydrogen-bonded, mixed-valent states are stabilized by −5.27 (0.04) kcal mol−1 relative to the isovalent, neutral, hydrogen-bonded dimers and −3.47 (0.06) kcal mol−1 relative to the isovalent, dianionic hydrogen bonded dimers. Electron exchange in the mixed valence states imparts significant stability to hydrogen bonding. This is the first quantitative measurement of the strength of hydrogen bonds in the presence and absence of electronic exchange.


 Please wait while we load your content...
Please wait while we load your content...