Highly selective acylation of polyamines and aminoglycosides by 5-acyl-5-phenyl-1,5-dihydro-4H-pyrazol-4-ones†
Abstract
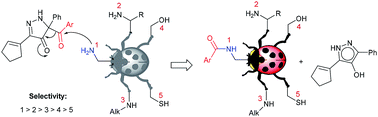
5-Acyl-5-phenyl-1,5-dihydro-4H-pyrazol-4-ones, accessible from arylpropargyl phenyldiazoacetates, are highly selective acyl transfer reagents for di- and polyamines, as well as aminoalcohols and aminothiols. As reagents with a carbon-based leaving group, they have been applied for benzoyl transfer with a broad selection of substrates containing aliphatic amino in combination with other competing nucleophilic functional groups. The substrate scope and levels of selectivity for direct benzoyl transfer exceed those of known benzoylating reagents. With exceptional selectivity for acylation between primary amines bound to primary and secondary carbons, these new reagents have been used in direct site-selective monobenzoylation of aminoglycoside antibiotics.


 Please wait while we load your content...
Please wait while we load your content...