Enantioselective Nazarov cyclization of indole enones cooperatively catalyzed by Lewis acids and chiral Brønsted acids†
Abstract
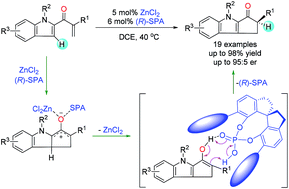
Enantioselective control of the chirality of a tertiary α-carbon in the products of a Nazarov cyclization of enones is challenging because the reaction involves an enantioselective proton transfer process. We herein report the use of cooperative catalysis using Lewis acids and chiral Brønsted acids to control the stereochemistry of the tertiary α-carbon in the products of this reaction. Specifically, with ZnCl2 and a chiral spiro phosphoric acid as catalysts, we realized the first enantioselective construction of cyclopenta[b]indoles with chiral tertiary α-carbons via Nazarov cyclization of indole enone substrates with only one coordinating site. Mechanistic studies revealed that the chiral spiro phosphoric acid acts as a multifunctional catalyst: it co-catalyzes the cyclization of the dienone and enantioselectively catalyzes a proton transfer reaction of the enol intermediate. This new strategy of enantioselective control by means of cooperative catalysis may show utility for other challenging asymmetric cyclization reactions.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...