Stereochemistry and biological activity of chlorinated lipids: a study of danicalipin A and selected diastereomers†
Abstract
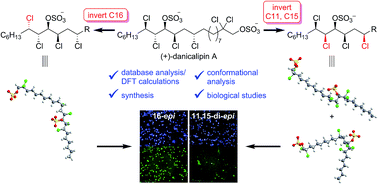
The syntheses of (+)-16-epi- and (+)-11,15-di-epi-danicalipin A (2 and 3) are reported. The conformations of the parent diols 5 and 6 as well as the corresponding disulfates 2 and 3 were determined on the basis of J-based configuration analysis and supported by calculations. The impact of configuration on membrane permeability in Gram-negative bacteria and mammalian cell lines was assessed as well as cytotoxicity. Although diastereomer 2 showed similar behavior to natural (+)-danicalipin A (1), strikingly, the more flexible C11,C15-epimer 3 had no effect on permeability and proved equally or more toxic towards multiple cell lines.


 Please wait while we load your content...
Please wait while we load your content...