Room temperature C(sp2)–H oxidative chlorination via photoredox catalysis†
Abstract
Photoredox catalysis has been developed to achieve oxidative C–H chlorination of aromatic compounds using NaCl as the chlorine source and Na2S2O8 as the oxidant. The reactions occur at room temperature and exhibit exclusive selectivity for C(sp2)–H bonds over C(sp3)–H bonds. The method has been used for the chlorination of a diverse set of substrates, including the expedited synthesis of key intermediates to bioactive compounds and a drug.
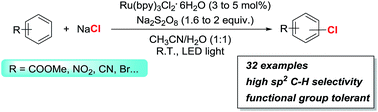


 Please wait while we load your content...
Please wait while we load your content...