Sortase-mediated chemical protein synthesis reveals the bidentate binding of bisphosphorylated p62 with K63 diubiquitin†
Abstract
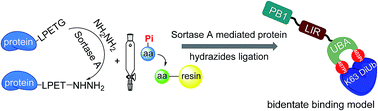
Phosphorylation of S403 or S407 of the autophagic receptor protein p62 has recently been discovered to enhance the binding of p62 with ubiquitinated protein substrates to upregulate selective autophagy. To elucidate the molecular mechanism of how phosphorylation regulates the recruitment of ubiquitinated proteins, we report the first chemical synthesis of homogeneously phosphorylated p62, which enables the setting up of accurate in vitro systems for biochemical studies. Our synthesis employs the technology of sortase A-mediated protein hydrazide ligation, which successfully affords three types of phosphorylated p62 at the multi-milligram scale. Quantitative biochemical measurements show that the binding affinity of S403/S407-bisphosphorylated p62 to K63 diubiquitin is significantly higher than that of mono-phosphorylated p62. This finding suggests that phosphorylated S403 and S407 sites should bind to different epitopes on the ubiquitin chain. Furthermore, glutamate mutation is found to give a significantly impaired binding affinity, implying the necessity of using chemically synthesized phosphorylated p62 for the biochemical study of selective autophagy.


 Please wait while we load your content...
Please wait while we load your content...