HOMO inversion as a strategy for improving the light-absorption properties of Fe(ii) chromophores†
Abstract
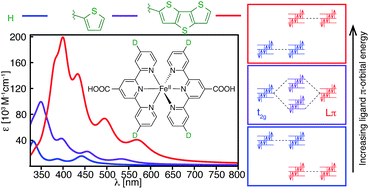
A computational study of a series of [Fe(tpy)2]2+ (tpy = 2,2′:6′,2′′-terpyridine) complexes is reported, where the tpy ligand is substituted at the 4, 4′, and 4′′ positions by electron donor (furan, thiophene, selenophene, NH2) and acceptor (carboxylic acid, NO2) groups. Using DFT and TD-DFT calculations, we show that the substitution of heterocyclic π donor groups onto the tpy ligand scaffold leads to marked improvement of the [Fe(tpy)2]2+ absorption properties, characterized by increased molar extinction coefficients, shift of absorption energies to longer wavelengths, and broadening of the absorption spectrum in the visible region. The observed changes in the light absorption properties are due to destabilization of ligand-centered occupied π orbital energies, thus increasing the interactions between the metal t2g (HOMO) and ligand π orbitals. Substitution of extended π-conjugated groups, such as thienothiophene and dithienothiophene, further destabilizes the ligand π orbital energies, resulting in a fully ligand-localized HOMO (i.e., HOMO inversion) and additional improvement of the light absorption properties. These results open up a new strategy to tuning the light absorption properties of Fe(II)-polypyridines.


 Please wait while we load your content...
Please wait while we load your content...