Copper-catalyzed cyanothiolation to incorporate a sulfur-substituted quaternary carbon center†
Abstract
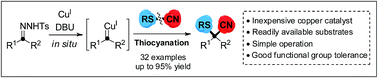
Sulfur-containing nitriles have important research value in the life sciences due to their diverse biological activities resulting from the sulfur and cyano functional groups. Herein, a copper-catalyzed cyanothiolation of N-tosylhydrazones with thiocyanates to generate α-arylthioalkanenitriles bearing sulfur-substituted quaternary carbon center atoms has been described. This novel protocol involves the procedure of copper carbene species promoting S–CN bond cleavage and C–CN/C–S bond reconstruction to introduce both sulfur and cyano groups onto a single carbon center. This cyanothiolation reaction will greatly enhance the synthetic utility of carbenoid species as new entries for the construction of diverse heteroatom-containing nitriles via cyanofunctionalization of metal–carbene species.


 Please wait while we load your content...
Please wait while we load your content...