Reduction rate as a quantitative knob for achieving deterministic synthesis of colloidal metal nanocrystals
Abstract
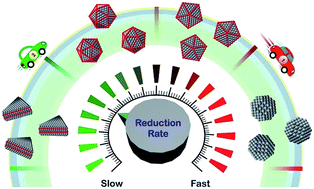
Despite the incredible developments made to the synthesis of colloidal metal nanocrystals, it is still challenging to produce them in a reproducible and predictable manner. This drawback can be attributed to the fact that the protocols continue to be built upon qualitative observations and empirical laws. Because of the vast number of intricately entangled experimental parameters in a synthesis, it is almost impossible to predict and control the outcome by knowingly alternating these parameters. In this Perspective article, we discuss the recent efforts in pushing nanocrystal synthesis towards a deterministic process based upon quantitative measurements. In particular, we focus on how the reduction rate of a salt precursor can be used as a quantitative knob for predicting and controlling the outcomes of both nucleation and growth. We begin with a brief introduction to the techniques that have been used to extract the kinetic information of a synthesis and then discuss how the reduction rate is correlated with the defect structure, shape/morphology, and elemental distribution of the resultant nanocrystals. We conclude by highlighting some of the recent advances related to in situ probing of nanocrystal synthesis, with an emphasis on the real-time, quantitative aspects with regard to both nucleation and growth.


 Please wait while we load your content...
Please wait while we load your content...