Michael addition-based probes for ratiometric fluorescence imaging of protein S-depalmitoylases in live cells and tissues†
Abstract
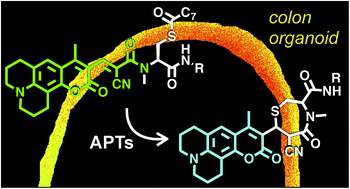
The reversible modification of cysteine residues through thioester formation with palmitate (protein S-palmitoylation) is a prevalent chemical modification that regulates the function, localization, and stability of many proteins. Current methods for monitoring the “erasers” of S-palmitoylation, acyl-protein thioesterases (APTs), rely on destructive proteomic methods or “turn-on” probes, precluding deployment in heterogeneous samples such as primary tissues. To address these challenges, we present the design, synthesis, and biological evaluation of Ratiometric Depalmitoylation Probes (RDPs). RDPs respond to APTs with a robust ratiometric change in fluorescent signal both in vitro and in live cells. Moreover, RDPs can monitor endogenous APT activities in heterogeneous primary human tissues such as colon organoids, presaging the utility of these molecules in uncovering novel roles for APTs in metabolic regulation.


 Please wait while we load your content...
Please wait while we load your content...