Diastereodivergent asymmetric Michael-alkylation reactions using chiral N,N′-dioxide/metal complexes†
Abstract
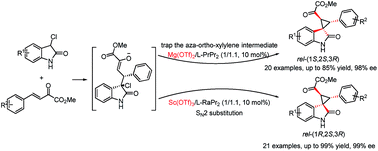
A diastereodivergent asymmetric Michael-alkylation reaction between 3-chloro-oxindoles and β,γ-unsaturated-α-ketoesters has been achieved using L-RaPr2/Sc(OTf)3 and L-PrPr2/Mg(OTf)2 metal complexes as catalysts. Both rel-(1R,2S,3R) and rel-(1S,2S,3R) chiral spiro cyclopropane oxindoles were constructed in good yields, diastereoselectivities and ee values. The diastereodivergent control may originate from different alkylation pathways after the Michael addition, with either intramolecular trapping of the aza-ortho-xylylene intermediate or direct SN2 substitution.


 Please wait while we load your content...
Please wait while we load your content...