Semisynthetic prion protein (PrP) variants carrying glycan mimics at position 181 and 197 do not form fibrils†
Abstract
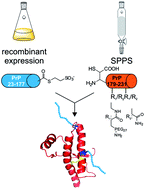
The prion protein (PrP) is an N-glycosylated protein attached to the outer leaflet of eukaryotic cell membranes via a glycosylphosphatidylinositol (GPI) anchor. Different prion strains have distinct glycosylation patterns and the extent of glycosylation of potentially pathogenic misfolded prion protein (PrPSc) has a major impact on several prion-related diseases (transmissible spongiform encephalopathies, TSEs). Based on these findings it is hypothesized that posttranslational modifications (PTMs) of PrP influence conversion of cellular prion protein (PrPC) into PrPSc and, as such, modified PrP variants are critical tools needed to investigate the impact of PTMs on the pathogenesis of TSEs. Here we report a semisynthetic approach to generate PrP variants modified with monodisperse polyethyleneglycol (PEG) units as mimics of N-glycans. Incorporating PEG at glycosylation sites 181 and 197 in PrP induced only small changes to the secondary structure when compared to unmodified, wildtype PrP. More importantly, in vitro aggregation was abrogated for all PEGylated PrP variants under conditions at which wildtype PrP aggregated. Furthermore, the addition of PEGylated PrP as low as 10 mol% to wildtype PrP completely blocked aggregation. A similar effect was observed for synthetic PEGylated PrP segments comprising amino acids 179–231 alone if these were added to wildtype PrP in aggregation assays. This behavior raises the question if large N-glycans interfere with aggregation in vivo and if PEGylated PrP peptides could serve as potential therapeutics.


 Please wait while we load your content...
Please wait while we load your content...