Ketones as directing groups in photocatalytic sp3 C–H fluorination†
Abstract
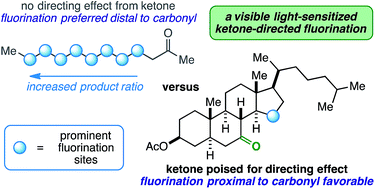
The ubiquitous ketone carbonyl group generally deactivates substrates toward radical-based fluorinations, especially sites closest to it. Herein, ketones are used instead to direct aliphatic fluorination using Selectfluor, catalytic benzil, and visible light. Selective β- and γ-fluorination are demonstrated on rigid mono-, di-, tri-, and tetracyclic (steroidal) substrates employing both cyclic and exocyclic aliphatic ketones as directing groups.


 Please wait while we load your content...
Please wait while we load your content...