Suzuki–Miyaura cross-coupling of amides and esters at room temperature: correlation with barriers to rotation around C–N and C–O bonds†
Abstract
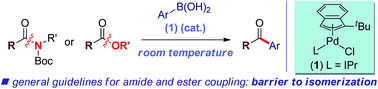
The Suzuki–Miyaura cross-coupling has been widely recognized as one of the most important methods for the construction of C–C bonds. However, in contrast to traditional aryl halide or pseudohalide electrophiles, coupling reactions with unactivated C–N and C–O electrophiles have proven significantly more challenging. Here we report the first general palladium-catalyzed Suzuki–Miyaura cross-coupling of both common amides and aryl esters through the selective cleavage of the C–N and C–O bonds under exceedingly mild conditions. Notably, for the first time we demonstrate selective C(acyl)–N and C(acyl)–O cleavage/cross-coupling under the same reaction conditions. The reaction uses a commercially available, bench-stable and operationally-convenient (η3-1-t-Bu-indenyl)Pd(IPr)(Cl) precatalyst. Furthermore, we demonstrate that the reactivity of generic amides and aryl esters can be correlated with barriers to isomerization around the C(acyl)–X (X = N, O) bond, thus providing a blueprint for the development of a broad range of novel coupling reactions of ester and amide electrophiles by the selective activation of C–O and C–N bonds.
- This article is part of the themed collection: Most downloaded articles of 2017: Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...