What is the fundamental ion-specific series for anions and cations? Ion specificity in standard partial molar volumes of electrolytes and electrostriction in water and non-aqueous solvents†
Abstract
The importance of electrolyte solutions cannot be overstated. Beyond the ionic strength of electrolyte solutions the specific nature of the ions present is vital in controlling a host of properties. Therefore ion specificity is fundamentally important in physical chemistry, engineering and biology. The observation that the strengths of the effect of ions often follows well established series suggests that a single predictive and quantitative description of specific-ion effects covering a wide range of systems is possible. Such a theory would revolutionise applications of physical chemistry from polymer precipitation to drug design. Current approaches to understanding specific-ion effects involve consideration of the ions themselves, the solvent and relevant interfaces and the interactions between them. Here we investigate the specific-ion effects trends of standard partial molar volumes and electrostrictive volumes of electrolytes in water and eleven non-aqueous solvents. We choose these measures as they relate to bulk properties at infinite dilution, therefore they are the simplest electrolyte systems. This is done to test the hypothesis that the ions alone exhibit a specific-ion effect series that is independent of the solvent and unrelated to surface properties. The specific-ion effects trends of standard partial molar volumes and normalised electrostrictive volumes examined in this work show a fundamental ion-specific series that is reproduced across the solvents, which is the Hofmeister series for anions and the reverse lyotropic series for cations, supporting the hypothesis. This outcome is important in demonstrating that ion specificity is observed at infinite dilution and demonstrates that the complexity observed in the manifestation of specific-ion effects in a very wide range of systems is due to perturbations of solvent, surfaces and concentration on the underlying fundamental series. This knowledge will guide a general understanding of specific-ion effects and assist in the development of a quantitative predictive theory of ion specificity.
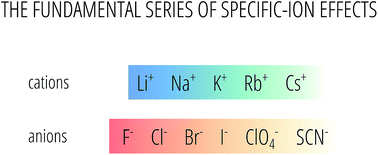


 Please wait while we load your content...
Please wait while we load your content...