A new face of phenalenyl-based radicals in the transition metal-free C–H arylation of heteroarenes at room temperature: trapping the radical initiator via C–C σ-bond formation†
Abstract
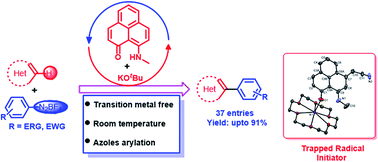
The radical-mediated transition metal-free approach for the direct C–H bond functionalization of arenes is considered as a cost effective alternative to transition metal-based catalysis. An organic ligand-based radical plays a key role by generating an aryl radical which undergoes a subsequent functionalization process. The design principle of the present study takes advantage of a relatively stable odd alternant hydrocarbon-based phenalenyl (PLY) radical. In this study, the first transition metal-free catalyzed direct C–H arylation of a variety of heteroarenes such as azoles, furan, thiophene and pyridine at room temperature has been reported using a phenalenyl-based radical without employing any photoactivation step. This protocol has been successfully applied to the gram scale synthesis of core moieties of bioactive molecules. The phenalenyl-based radical initiator has been characterized crystallographically by trapping it via the formation of a C–C σ-bond between the phenalenyl radical and solvent-based radical species.


 Please wait while we load your content...
Please wait while we load your content...