Electrochemical maps and movies of the hydrogen evolution reaction on natural crystals of molybdenite (MoS2): basal vs. edge plane activity†
Abstract
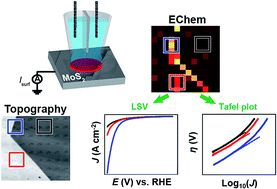
Two dimensional (2D) semiconductor materials, such as molybdenum disulfide (MoS2) have attracted considerable interest in a range of chemical and electrochemical applications, for example, as an abundant and low-cost alternative electrocatalyst to platinum for the hydrogen evolution reaction (HER). While it has been proposed that the edge plane of MoS2 possesses high catalytic activity for the HER relative to the “catalytically inert” basal plane, this conclusion has been drawn mainly from macroscale electrochemical (voltammetric) measurements, which reflect the “average” electrocatalytic behavior of complex electrode ensembles. In this work, we report the first spatially-resolved measurements of HER activity on natural crystals of molybdenite, achieved using voltammetric scanning electrochemical cell microscopy (SECCM), whereby pixel-resolved linear-sweep voltammogram (LSV) measurements have allowed the HER to be visualized at multiple different potentials to construct electrochemical flux movies with nanoscale resolution. Key features of the SECCM technique are that characteristic surface sites can be targeted and analyzed in detail and, further, that the electrocatalyst area is known with good precision (in contrast to many macroscale measurements on supported catalysts). Through correlation of the local voltammetric response with information from scanning electron microscopy (SEM) and atomic force microscopy (AFM) in a multi-microscopy approach, it is demonstrated unequivocally that while the basal plane of bulk MoS2 (2H crystal phase) possesses significant activity, the HER is greatly facilitated at the edge plane (e.g., surface defects such as steps, edges or crevices). Semi-quantitative treatment of the voltammetric data reveals that the HER at the basal plane of MoS2 has a Tafel slope and exchange current density (J0) of ∼120 mV per decade and 2.5 × 10−6 A cm−2 (comparable to polycrystalline Co, Ni, Cu and Au), respectively, while the edge plane has a comparable Tafel slope and a J0 that is estimated to be more than an order-of-magnitude larger (∼1 × 10−4 A cm−2). Finally, by tracking the temporal evolution of water contact angle (WCA) after cleavage, it is shown that cathodic polarization has a ‘self-cleaning’ effect on the surface of MoS2, consistent with the time-independent (i.e., time after cleavage) HER voltammetric response.


 Please wait while we load your content...
Please wait while we load your content...