A complementary pair of enantioselective switchable organocatalysts†
Abstract
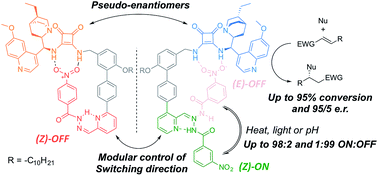
A pair of enantioselective switchable bifunctional catalysts are shown to promote a range of conjugate addition reactions in up to 95 : 5 e.r. and 95% conversion. Each catalyst can be switched OFF using conditions that switch the other catalyst ON. Catalyst ON : OFF ratios of up to 98 : 2 and 1 : 99 were achieved, with a ratio of reaction rates of up to 16 : 1 between the ON and OFF states, maintained over complete ON–OFF–ON and OFF–ON–OFF cycles. However, simultaneous operation of the catalyst pair in the same reaction vessel, which in principle could allow product handedness to be switched by simple E–Z isomerisation of the catalyst pair, was unsuccessful. In this first generation complementary pair of enantioselective switchable organocatalysts, the OFF state of one catalyst inhibits the ON state of the other.
- This article is part of the themed collection: International Symposium on Macrocyclic & Supramolecular Chemistry (ISMSC) in conjunction with ISACS


 Please wait while we load your content...
Please wait while we load your content...