Intramolecular substitution uncages fluorogenic probes for detection of metallo-carbapenemase-expressing bacteria†
Abstract
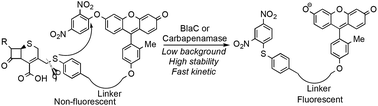
This work reports a novel caging strategy for designing fluorogenic probes to detect the activity of β-lactamases. The caging strategy uses a thiophenyl linker connected to a fluorophore caged by a good leaving group—dinitrophenyl. The uncaging proceeds in two steps through the sulfa-releasing and subsequent intramolecular substitution. The length of the linker has been examined and optimized to maximize the rate of intramolecular reaction and thus the rate of fluorescence activation. Finally based on this strategy, we prepared a green fluorogenic probe CAT-7 and validated its selectivity for detecting metallo-carbapenemases (VIM-27, IMP-1, NDM-1) in carbapenem-resistant Enterobacteriaceae (CRE) lysates.


 Please wait while we load your content...
Please wait while we load your content...