Identification of catabolite control protein A from Staphylococcus aureus as a target of silver ions†
Abstract
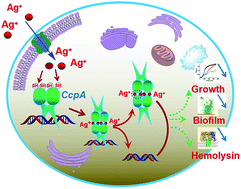
Staphylococcus aureus is one of the most common pathogenic bacteria that causes human infectious diseases. The emergence of antibiotic-resistant strains of S. aureus promotes the development of new anti-bacterial strategies. Silver ions (Ag+) have attracted profound attention due to their broad-spectrum antimicrobial activities. Although the antibacterial properties of silver have been well known for many centuries, its mechanism of action remains unclear and its protein targets are rarely reported. Herein, we identify the catabolite control protein A (CcpA) of S. aureus as a putative target for Ag+. CcpA binds 2 molar equivalents of Ag+via its two cysteine residues (Cys216 and Cys242). Importantly, Ag+ binding induces CcpA oligomerization and abolishes its DNA binding capability, which further attenuates S. aureus growth and suppresses α-hemolysin toxicity. This study extends our understanding of the bactericidal effects of silver.


 Please wait while we load your content...
Please wait while we load your content...