Borylation of fluorinated arenes using the boron-centred nucleophile B(CN)32− – a unique entry to aryltricyanoborates†
Abstract
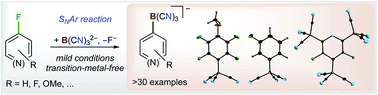
The potassium salt of the boron-centred nucleophile B(CN)32− (1) readily reacts with perfluorinated arenes, such as hexafluorobenzene, decafluorobiphenyl, octafluoronaphthalene and pentafluoropyridine, which results in KF and the K+ salts of the respective borate anions with one {B(CN)3} unit bonded to the (hetero)arene. An excess of K21 leads to the successive reaction of two or, in the case of perfluoropyridine, even three C–F moieties and the formation of di- and trianions, respectively. Moreover, all of the 11 partially fluorinated benzene derivatives, C6F6−nHn (n = 1–5), generally react with K21 to give new tricyano(phenyl)borate anions with high chemo- and regioselectivity. A decreasing number of fluorine substituents on benzene results in a decrease in the reaction rate. In the cases of partially fluorinated benzenes, the addition of LiCl is advantageous or even necessary to facilitate the reaction. Also, pentafluorobenzenes R–C6F5 (R = –CN, –OMe, –Me, or –CF3) react via C–F/C–B exchange that mostly occurs in the para position and to a lesser extent in the meta or ortho positions. Most of the reactions proceed via an SNAr mechanism. The reaction of 1,4-F2C6H4 with K21 shows that an aryne mechanism has to be considered in some cases as well. In summary, a wealth of new stable tricyano(aryl)borates have been synthesised and fully characterized using multi-NMR spectroscopy and most of them were characterised using single-crystal X-ray diffraction.


 Please wait while we load your content...
Please wait while we load your content...