Dual targeting of the cancer antioxidant network with 1,4-naphthoquinone fused Gold(i) N-heterocyclic carbene complexes†
Abstract
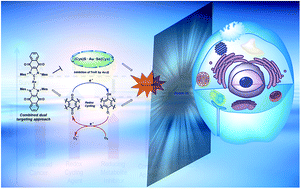
To achieve a systems-based approach to targeting the antioxidant pathway, 1,4-naphthoquinone annulated N-heterocyclic carbene (NHC) [bis(1,3-dimesityl-4,5-naphthoquino-imidazol-2-ylidene)-gold(I)] [silver(I) dichloride] (1), [bis(1,3-dimesityl-4,5-naphthoquino-imidazol-2-ylidene)-gold(I)] chloride (2), and 1,3-dimesityl-4,5-naphthoquino-imidazol-2-ylidene)-gold(I) chloride (3)) were designed, synthesized, and tested for biological activity in a series of human cancer cell lines. The solution phase of complexes 1–3 were assigned using several spectroscopy techniques, including NMR spectroscopic analysis. Complexes 1 and 3 were further characterized by single crystal X-ray diffraction analysis. Electrochemical and spectroelectrochemical studies revealed that quinone reductions are reversible and that the electrochemically generated semiquinone and quinone dianions are stable under these conditions. Complex 1, containing two NHC-quinone moieties (to accentuate exogenous ROS via redox cycling) centered around a Au(I) center (to inactivate thioredoxin reductase (TrxR) irreversibly), was found to inhibit cancer cell proliferation to a much greater extent than the individual components (i.e., Au(I)–NHC alone or naphthoquinone alone). Treatment of A549 lung cancer cells with 1 produced a 27-fold increase in exogenous reactive oxygen species (ROS) which was found to localize to the mitochondria. The inhibition of TrxR, an essential mediator of ROS homeostasis, was achieved in the same cell line at low administrated concentrations of 1. TrxR inhibition by 1 was similar to that of auranofin, a gold(I) containing complex known to inhibit TrxR irreversibly. Complex 1 was found to induce cell death via an apoptotic mechanism as confirmed by annexin-V staining. Complex 1 was demonstrated to be efficacious in zebrafish bearing A549 xenografts. These results provide support for the suggestion that a dual targeting approach that involves reducing ROS tolerance while concurrently increasing ROS production can perturb antioxidant homeostasis, enhance cancer cell death in vitro, and reduce tumor burden in vivo, as inferred from preliminary zebra fish model studies.


 Please wait while we load your content...
Please wait while we load your content...