Bioinspired enantioselective synthesis of crinine-type alkaloids via iridium-catalyzed asymmetric hydrogenation of enones†
Abstract
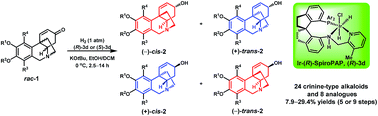
A bioinspired enantioselective synthesis of crinine-type alkaloids has been developed by iridium-catalyzed asymmetric hydrogenation of racemic cycloenones. The method features a biomimetic stereodivergent resolution of the substrates bearing a remote arylated quaternary stereocenter. Using this protocol, 24 crinine-type alkaloids and 8 analogues were synthesized in a concise and rapid way with high yield and high enantioselectivity.
- This article is part of the themed collection: Celebrating 100 Years of Chemistry at Nankai University


 Please wait while we load your content...
Please wait while we load your content...